Theory
Purification by size exclusion chromatography
In order to separate the protein mixture by size exclusion chromatography, the resin is packed into a column to form a packed bed. The resin consists of a porous matrix of chemically and physically stable spherical particles that exhibit inertness and minimize the adsorption of biomolecules. The column is equilibrated with a buffer which fills the pores of the matrix and the space in between the particles. The liquid inside the pores(stationary phase), is in equilibrium with the liquid (buffer or mobile phase) outside the particles. The protein sample is eluted isocratically as the buffer composition remains constant throughout the experiment. After sample application, the separation takes place as one column volume of buffer (equivalent to the volume of the packed bed) passes through the column. Protein molecules that are larger in size than the largest pores in the matrix cannot enter the pores and are eluted in the void volume as they pass directly through the column. The void volume is approximately equal to 30% of the total column volume. Protein molecules that can partially access the pores elute from the column in order of decreasing size. Small molecules of salts that have entered the pores usually elute right before one column volume of buffer has passed through the column.
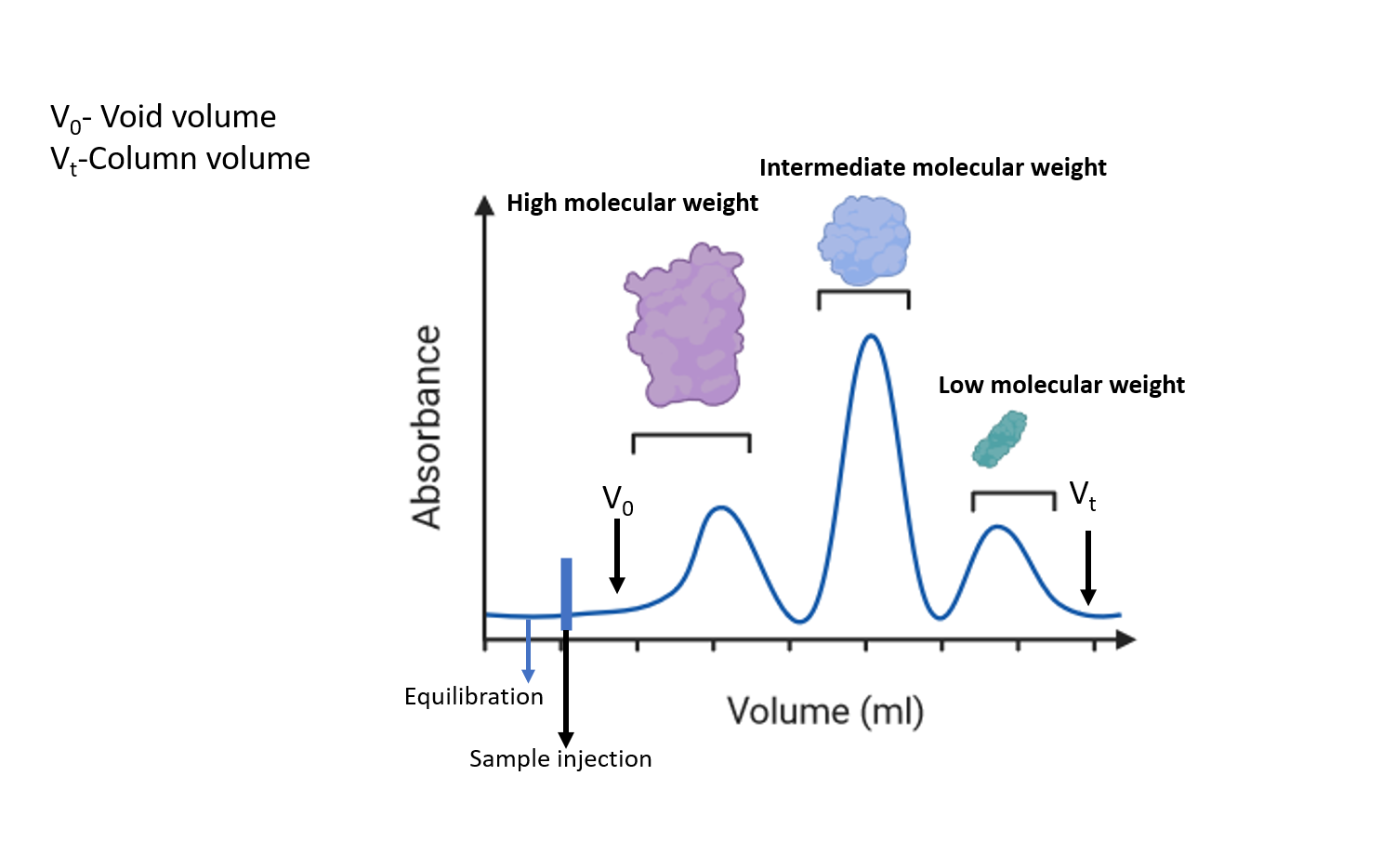
Figure 1 : Chromatogram representing size exclusion chromatography
Components of size exclusion chromatography column
Effective separation of the protein sample through the size exclusion chromatography column depends on a number of factors such as particle size, flow rate, packing density, and porosity of the column media. In order to optimize all these parameters, certain size exclusion chromatography media has been developed such as
- Sephadex : Sephadex is made by cross-linking dextran. It was one of the earliest size exclusion chromatography media developed and is used for desalting and buffer exchange of protein biomolecules.
- Superdex : Superdex is made by covalently linking dextran to highly cross-linked agarose particles. The media exhibits high physical and chemical stability, due to the presence of a highly cross-linked agarose matrix, and excellent chromatographic separation properties that are determined mainly by the dextran chains. For laboratory applications, it is the most preferable media with high-resolution fractionation with longer recovery of protein and shorter flow time.
- Superose: Superose is a media with high physical and chemical stability and it is made by cross-linking porous agarose particles.
- Sephacryl : Sephacryl is used generally as an alternative to superdex and is useful for separations that require a broad fractionation range. Sephacryl is made by covalently cross-linking allyl dextran and N, N’-Methylene bisacrylamide to provide a hydrophilic resin with minimized nonspecific adsorption.
Application of size exclusion chromatography :-
- Preparative size exclusion chromatography :- It is a high-resolution size-based separation mainly used for isolating one or more components of protein sample. Small sample volumes of 0.5% to 4% of the total column volume are applied at low flow rates using long columns. It is mainly the final polishing step in the protein purification procedure preceded by other chromatographic methods such as ion-exchange, affinity, hydrophobic chromatography etc. In order to achieve high resolution separation, a well-packed column is a necessity since the separation takes place in one column volume buffer.
- Analytical size exclusion chromatography :- It is used to study properties of protein biomolecules , check the purity of the sample, evaluate protein stability and study complex formation. Small sample volumes of 0.3% to 0.5% of the total column volume are applied at low flow rates using short columns. The resolution of separation is less than preparative size exclusion chromatography.
- Desalting and buffer exchange :- It is a group separation process during which small molecules such as salt or free labels are separated from a group of larger molecules such as proteins. Large sample volumes up to 30% of the total column volume can be applied at high flow rates using broad, short columns.




