



pH-titration of amino acids and small peptides, and estimation of isoelectric point (pI)
Amino acids are amphoteric. They are made of both ionizable acidic (α-carboxylic group) and basic (α-amino group) moieties. Therefore, they can act as acids as well as an alkali depending on the pH of the media.
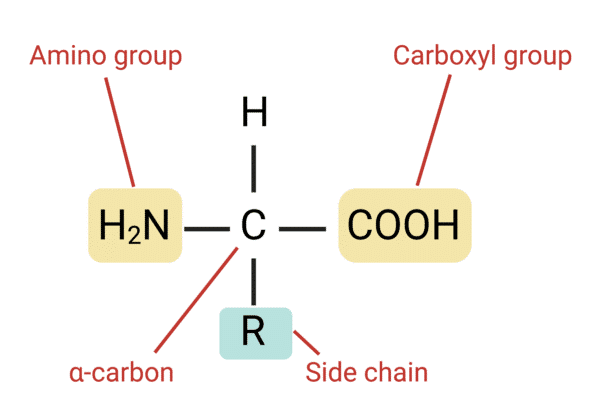
Figure 1: Chemical structure of an amino acid
Based on the properties of the side chain, the amino acids are classified into various types (Figure 2).
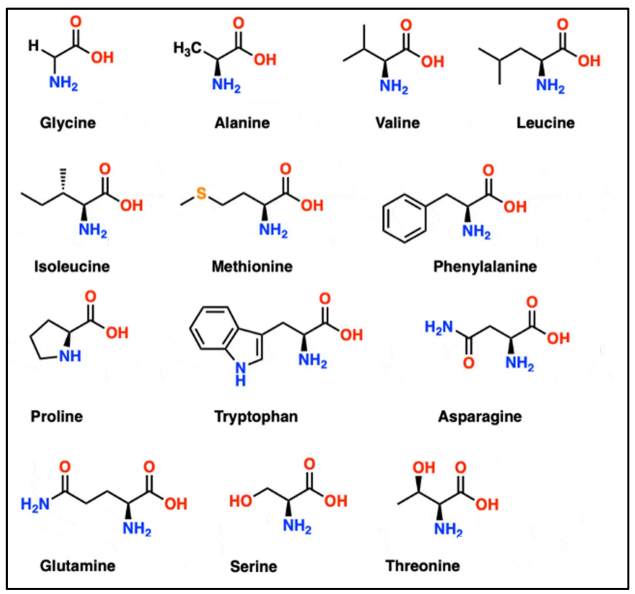
Figure 2: Structure of 20 common naturally occurring amino acids
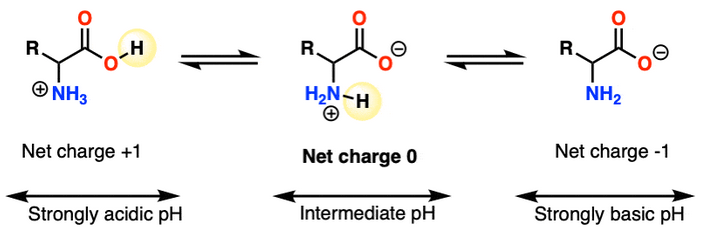
Figure 3: A typical amino acid with neutral R-group transitions from positively charged to a zwitterion and then to a negatively charged moiety as pH increases
The zwitterion is a salt formed as a result of the acid-base reactions of the basic amino group and acidic carboxylic group. The acid-base reactions are the following:
| Amino Acid | Side Chain | pKa1 (α- COOH) |
pKa2 (α-NH 3+) |
pKR (R-group) |
pI | Properties |
|---|---|---|---|---|---|---|
| Glycine (G) | H | 2.34 | 9.60 | - | 5.97 | Neutral |
| Alanine (A) | CH3 | 2.34 | 9.69 | - | 6.015 | Neutral |
| Valine (V) | (CH3)2CH | 2.32 | 9.62 | - | 6.48 | Neutral |
| Leucine (L) | (CH3)2CHCH2 | 2.36 | 9.60 | - | 5.98 | Neutral |
| Isoleucine (I) | CH3CH2CH(CH3) | 2.36 | 9.68 | - | 6.02 | Neutral |
| Serine (S) | CH2OH | 2.21 | 9.15 | - | 5.68 | Polar, Hydrophilic |
| Threonine (T) | CH(OH)CH3 | 2.09 | 9.10 | - | 5.595 | Polar, Hydrophilic |
| Cysteine (C) | SH | 1.96 | 8.18 | - | 5.07 | Polar, can form disulfide bonds |
| Methionine (M) | SCH3 | 2.28 | 9.21 | - | 5.74 | Nonpolar |
| Proline (P) | Fused to α-N | 1.99 | 10.60 | - | 6.295 | Unique structure, cyclic |
| Phenylalanine (F) | C6H5 | 1.83 | 9.13 | - | 5.48 | Nonpolar |
| Tyrosine (Y) | C6H4OH | 2.20 | 9.11 | 10.07 | 5.655 | Polar, Hydrophilic |
| Tryptophan (W) | C8H6NCH2 | 2.83 | 9.39 | - | 5.88 | Nonpolar |
| Histidine (H) | C5H4NCH | 1.82 | 9.17 | 6.00 | 7.585 | Positively charged at neutral pH |
| Glutamine (Q) | CH2CONH2 | 2.17 | 9.13 | - | 5.65 | Polar, Hydrophilic |
| Asparagine (N) | CH2CONH2 | 2.02 | 8.80 | - | 5.41 | Polar, Hydrophilic |
| Glutamic acid (E) | CH2CH2COOH | 2.19 | 9.67 | 4.25 | 3.22 | Negatively charged at neutral pH |
| Aspartic acid (D) | CH2COOH | 1.88 | 9.60 | 3.65 | 2.765 | Negatively charged at neutral pH |
| Lysine (K) | CH2CH2CH2NH3+ | 2.18 | 8.95 | 10.53 | 9.74 | Positively charged at neutral pH |
| Arginine (R) | C4H6N3+(NH)2 | 2.17 | 9.04 | 12.48 | 10.76 | Positively charged at neutral pH |
Table 1: The pka values of common α-amino acids grouped based on their charge, polarity, and side chain properties. (Ref: Adapted from Voet, D. and Voet, J.G. (2010) Biochemistry. 4th Edition, Wiley, Hoboken.)
How is the isoelectric point of an amino acid determined?
According to Henderson Hesselbalch equation (Figure 4), the pH equals pKa (the quantitative measure of the strength of an acid) when the concentration of the acid (HA) is the same as that of its conjugate base (A-). Lower pKa values mean a stronger acid. The pKa is the pH at the mid-point of the flat buffering zones in a titration plot (pH vs volume of titrant). Table 1 lists out the pKa and pI values of each amino acid along with their properties.
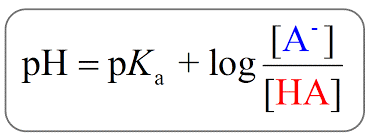
Figure 4: Henderson Hesselbalch equation
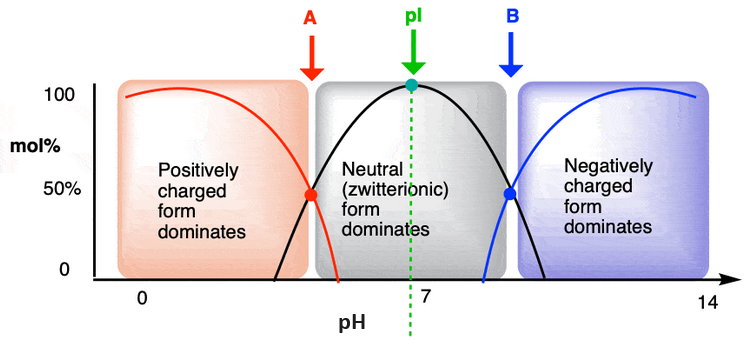
Figure 5: The pI lies between the points A and B where the concentration of an acid is the same as that of its conjugate base
To calculate the pI of a neutral amino acid, an average of the pKa1 and pKa2 values are taken. However, to find the pI of a triprotic acid, the average of the pKa values that are closest to the pH at which the amino acid assumes zero net charge is used (Figure 6).
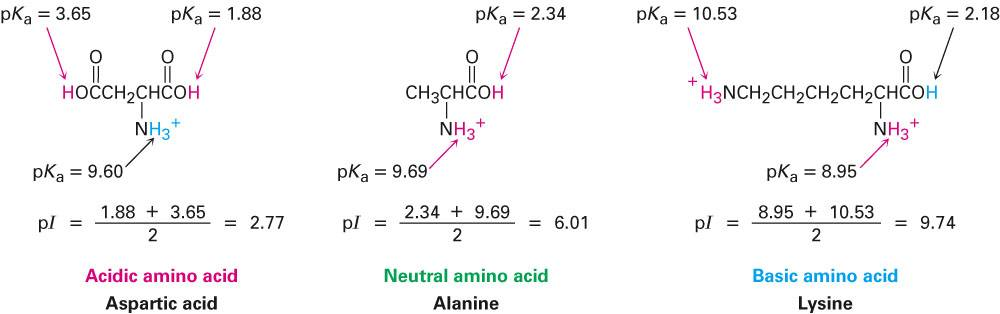
Figure 6: Calculation of the isoelectric point of amino acids
How is the isoelectric point of a peptide is determined?
The isoelectric point of a peptide can be determined in a similar fashion as described above. Let us take the example of a tripeptide Asp-Gly-Glu. The pKa values are listed below. As the pH increases from 1 to 10, the tripeptide transitions from net positive to negative charge. At pH above 2.2, net charge is zero. Therefore, the major contributors of the isoelectric point are the pKa values closest to pH 2.2 i.e. pKa 3.86 and 2.19. Therefore pI is calculated as an average of these 2 pKa values.

| pH | 1 | 2.5 | 4 | 5 | 10 |
|---|---|---|---|---|---|
| Net Charge | +1 | 0 | -1 | -2 | -3 |
Since zero net charge lies between pH of 2.19 and 3.86,
Isoelectric point = (2.19 + 3.86)/2 = 3.03
What is the biological and physiological significance of the amino acid titration curves?
Understanding the titration curves of amino acids reaches far and wide within the realm of biology and medicine. The knowledge of pH-dependent charge of the constituent amino acids of a protein allows researchers to predict the folding, stability, and interaction of proteins, and unravel deeper insights into enzyme mechanisms.
From a clinical perspective, understanding the impact of pH alternations on proteins can influence diagnostic and treatment strategies. A notable example is the disease metabolic acidosis, where a lower blood pH could potentially affect the structure and function of essential proteins. These studies find applications in nutrition and hereditary diseases as they throw light on why some mutations cause disease and others do not or on developing therapeutic strategies that alter protein interactions.