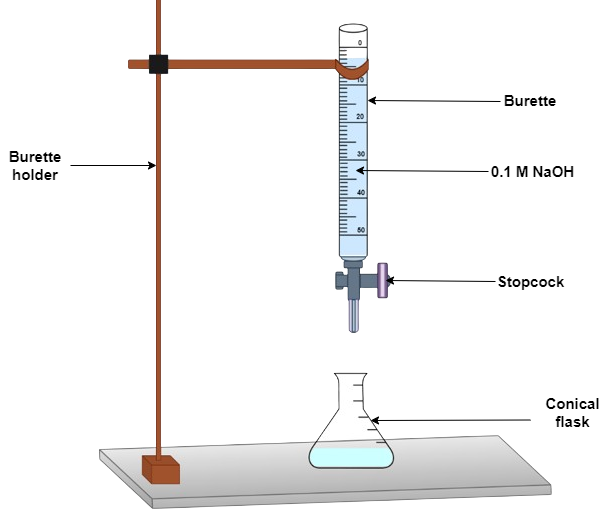
pH-titration of amino acids and small peptides, and estimation of isoelectric point (pI)
PART A: Titration of amino acids to determine the isoelectric point.
Step 1: Pipette 10 ml of 0.1 M amino acid solution (such as alanine) into a 50 ml conical flask.
Step 2: Titrate 0.5 ml of 0.1 M HCl from the burette into the amino acid solution and determine the pH of the solution after each addition.
Step 3: Continue adding acid in until pH falls to about 1.3. Record the pH.
Step 4: Wash the electrode in distilled water and titrate 10 ml of alanine solution with 0.1 M NaOH until pH reaches 12.5.
Step 5: Plot a titration curve for alanine as pH versus volume of NaOH used for titration (in ml) or [OH-] equivalence.
Step 6: Similarly, do carry out the titration of arginine (or any other triprotic acid) and plot the titration curve.
Step 7: Determine the pKa and pI values from the plots and compare them with the standard values.
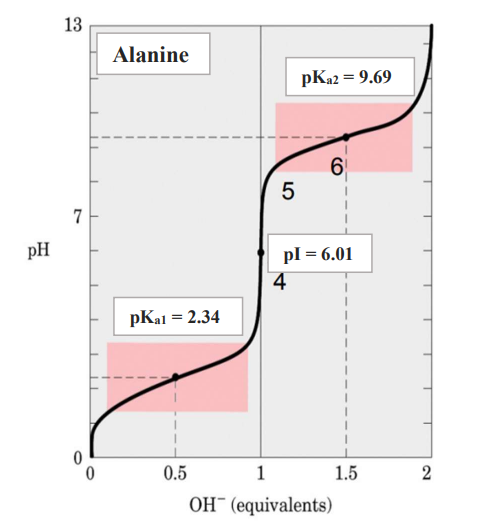
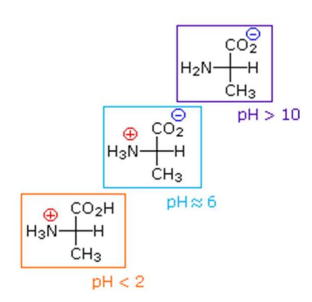
Titration curve for a diprotic amino acid (Alanine)
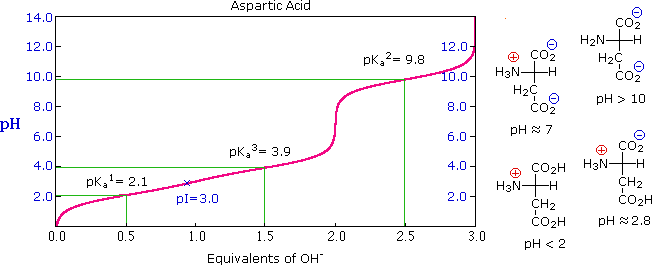
Titration curve for a triprotic acidic amino acid (Aspartic acid)
PART B: Titration of peptides to determine the isoelectric point.
The same procedure can be carried out to determine the isoelectric point of peptides.
Determine the isoelectric point of the tripeptide
Step 1: Write out the pKa values of the amino acid from low to high.
Step 2: Drop the pH below the lowest pKa value of the amino acid and determine the net charge of the amino acid.
Step 3: Select a pH between the first and the second pKa value of the amino acid and determine the net charge of the amino acid.
Step 4: Select a pH between the second and the third pKa value of the amino acid and determine the net charge of the amino acid.
Step 5: Raise the pH above the highest pKa value and determine the net charge of the amino acid.
Step 6: Calculate the pI by averaging the two pKa values that are just before and just after the zero net charge.
pH measurement
Titration