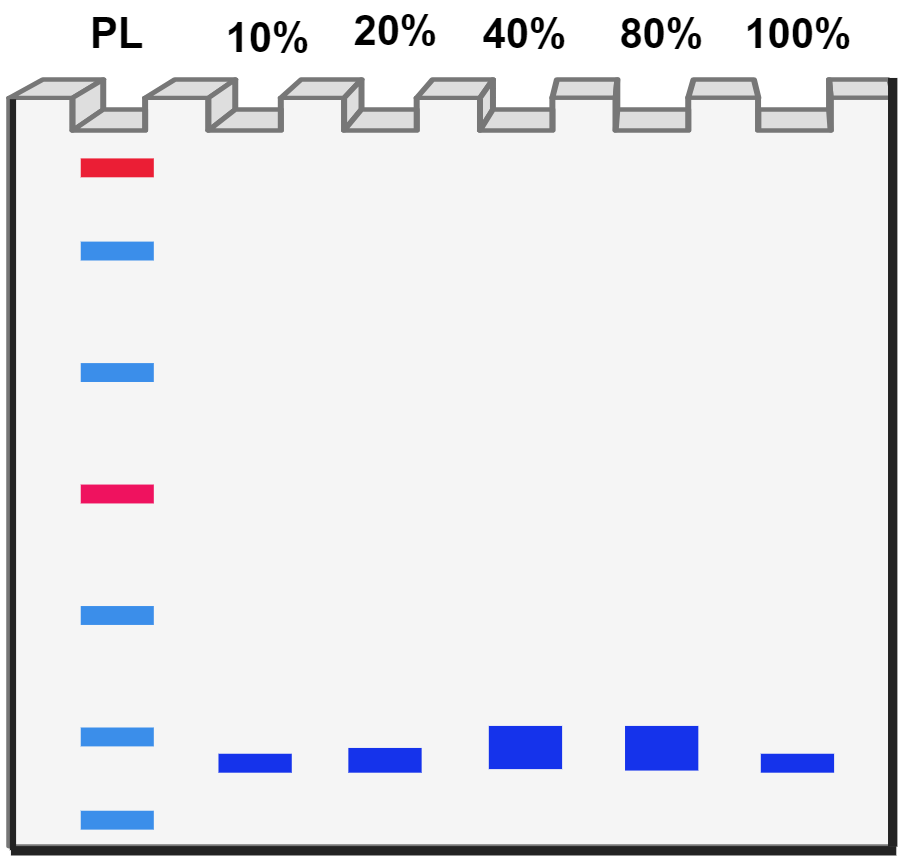
Protein purification by affinity chromatography
| Step 1 |
|
| Step 2 |
|
| Step 3 |
|
| Step 4 |
|
| Step 5 | |
| Step 6 | |
Step 1


Step 2
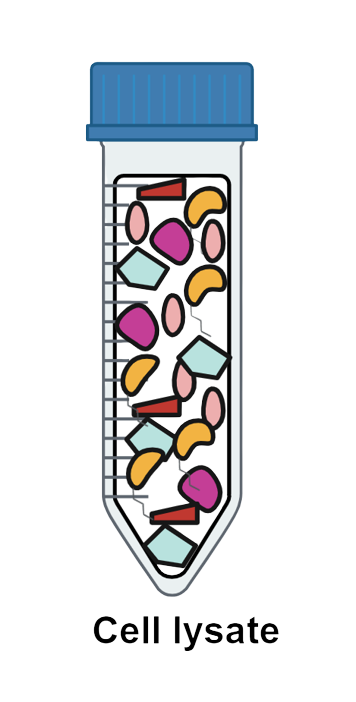
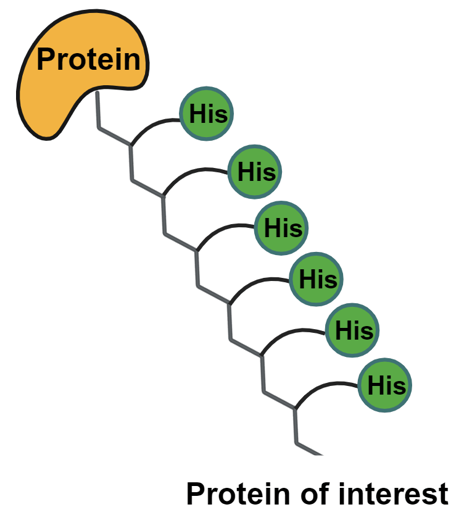

Step 3
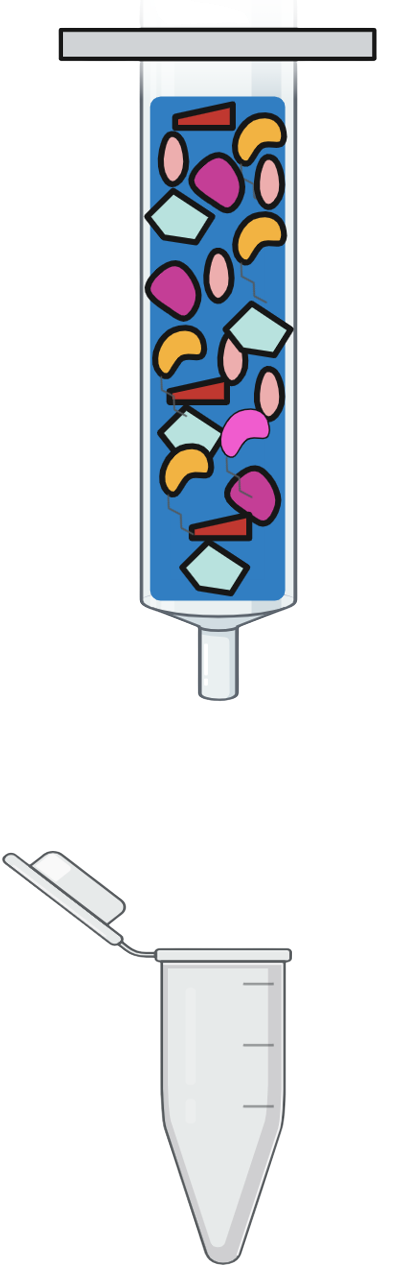

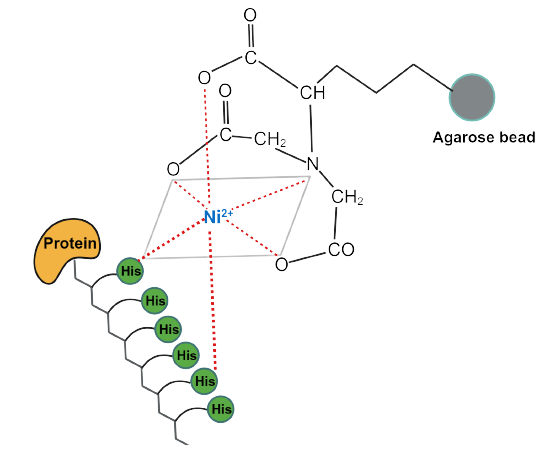

Step 4

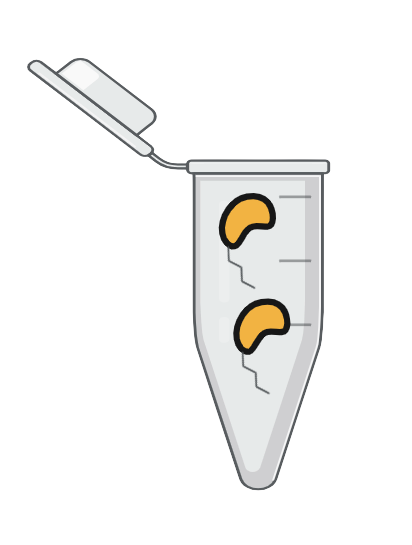
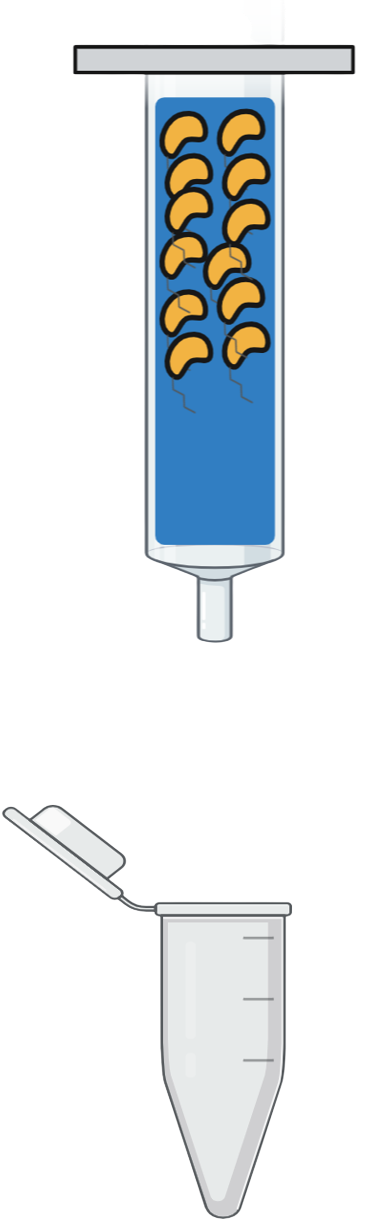
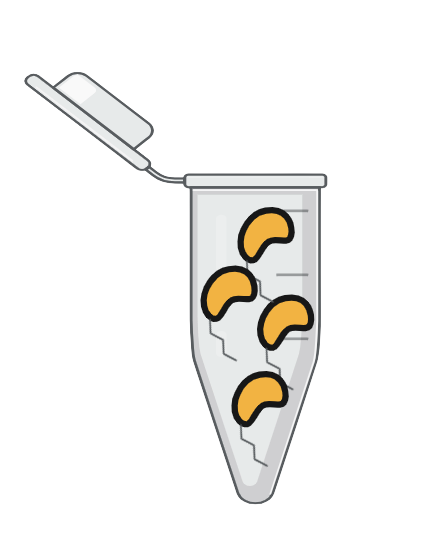
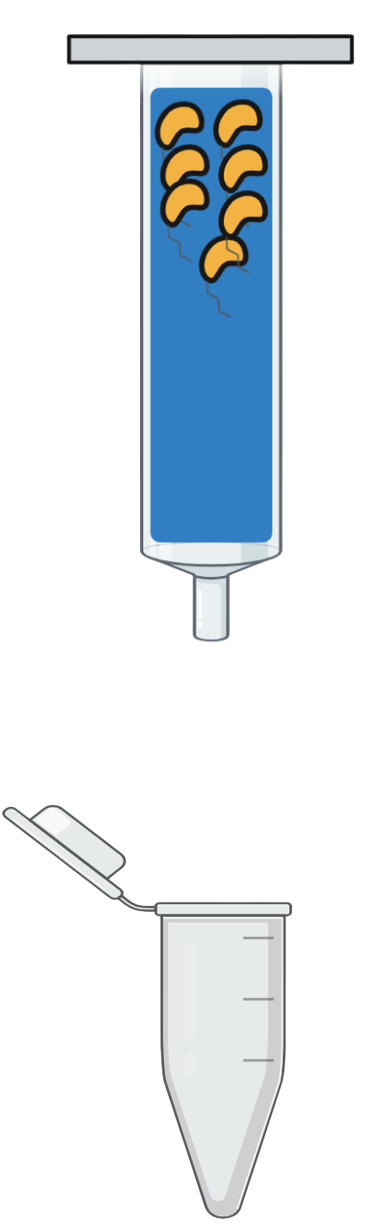
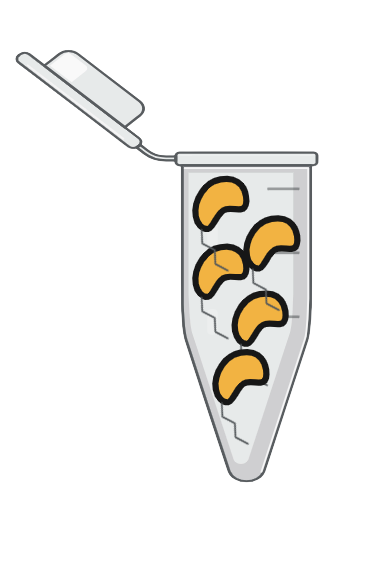
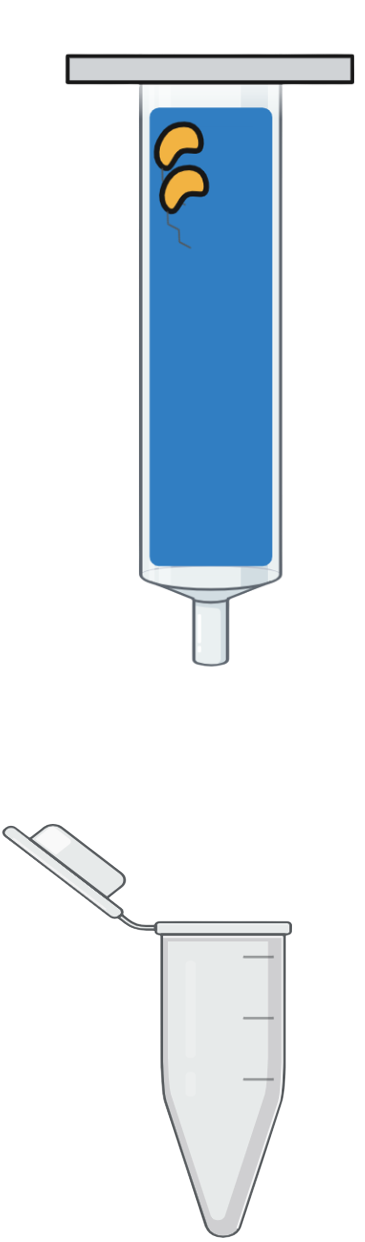

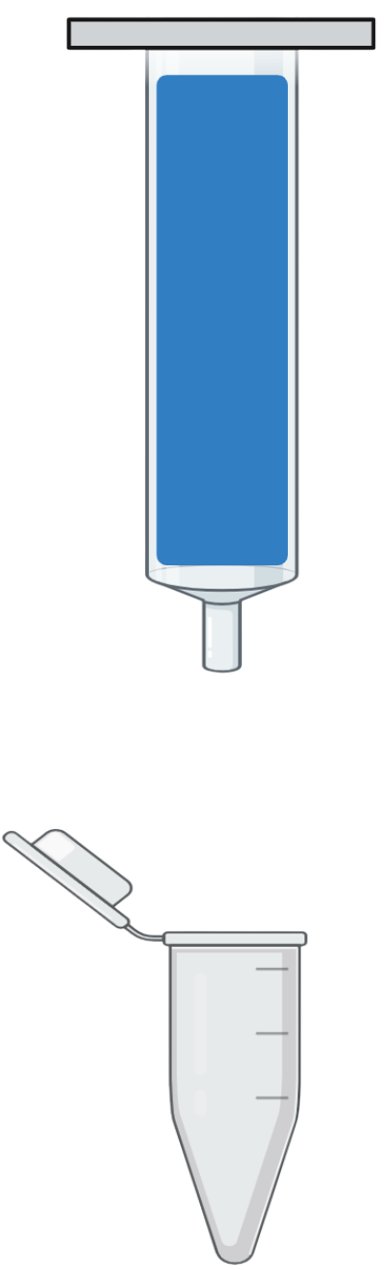
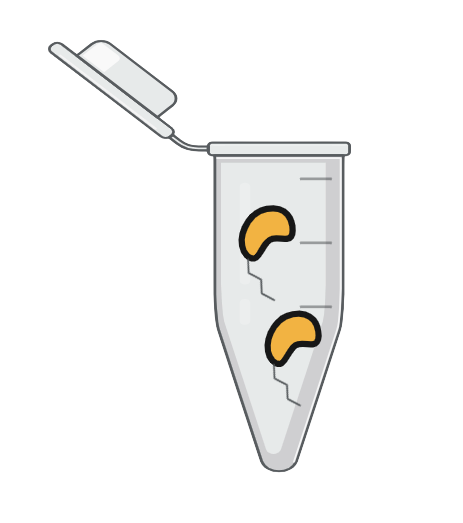

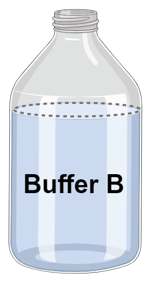
Step 5
Step 6