Procedure
In order to perform protein purification by affinity chromatography, the following steps must be followed:-
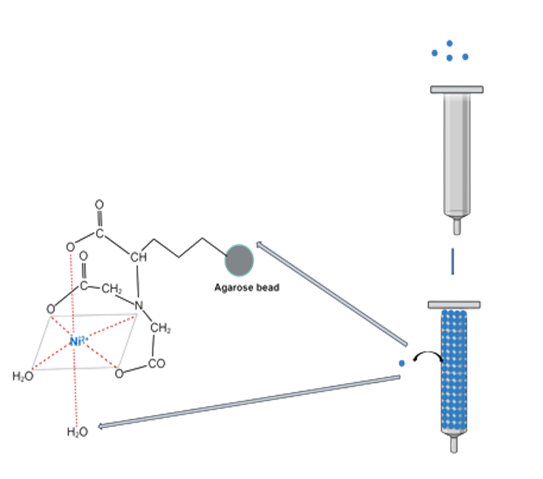
Preparing Ni-NTA Column (Equilibriation Step)
NOTE: One can use a pre-packed Ni-NTA column or can pack it manually.
When preparing a column as described below, make sure that the snap-off cap at the bottom of the column remains intact. To prepare a column:
- Resuspend the Ni-NTA Agarose in its bottle by inverting and gently tapping the bottle repeatedly.
- Pipet or pour 1.5 ml of the resin into a 10-ml Purification Column. Allow the resin to settle completely by gravity (5–10 minutes) or gently pellet it by low-speed centrifugation (1 minute at 800 × g). Gently aspirate the supernatant.
- Add 6 ml sterile, distilled water and resuspend the resin by alternately inverting and gently tapping the column.
- Allow the resin to settle using gravity or centrifugation as described in Step 2, and gently aspirate the supernatant.
- Add 6 ml Buffer A (buffer used for the lysis of the cells).
- Resuspend the resin by alternately inverting and gently tapping the column.
- Allow the resin to settle using gravity or centrifugation as described in Step 2, and gently aspirate the supernatant.
- Repeat Steps 5 through 7.
Storing Prepared Columns
To store a column containing resin either manually packed or pre-packed, add 20% ethanol as a preservative and cap or parafilm the column. Storing of column should be at 4°C.
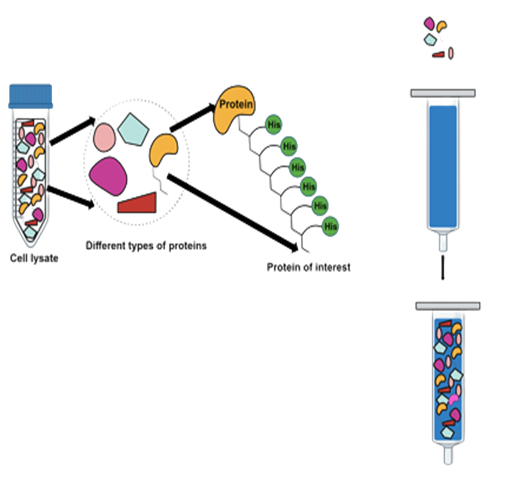
Purification of proteins
Using the native buffers, columns and cell lysate, follow the procedure below to purify proteins under native conditions:
- Add 5-10 ml cell lysate in a manually prepared or pre-packed Ni-NTA purification column.
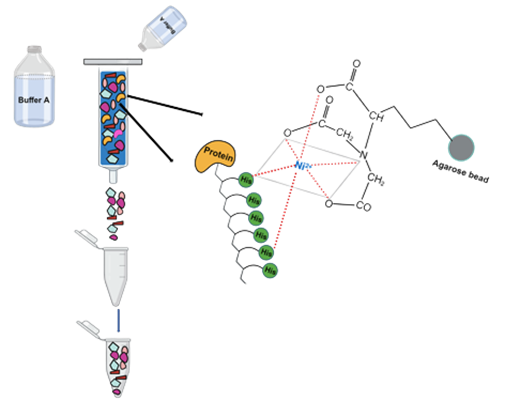
- Pass the Buffer A in the column at a speed of 2 ml/min and incubate for 5-10 minutes for proper binding of the proteins.
- Settle the resin by gravity or low-speed centrifugation (800 × g), and carefully aspirate the supernatant in case of a manually prepared column. Save supernatant at 4°C for SDS-PAGE analysis.
- Load the remaining cell lysate same as mentioned in Step (1-3).
- Wash the column with Buffer A (2ml/min) for 30-40 minutes.
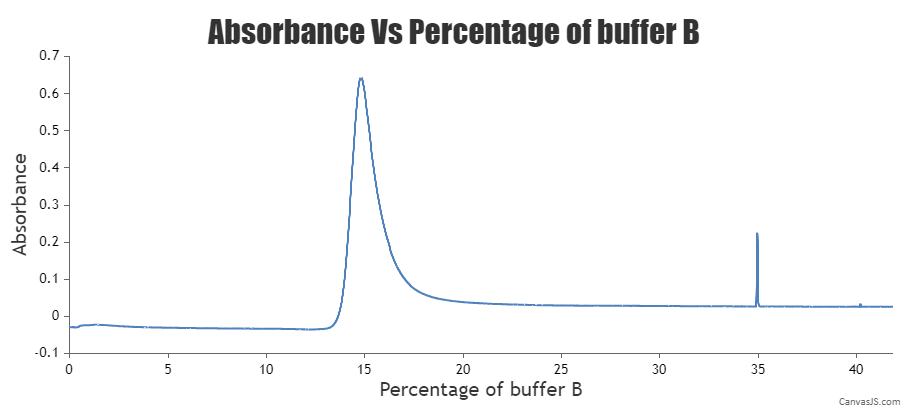
- Elute the protein of interest by passing the Elution Buffer (Buffer B) in a gradient. Collect 1-2 ml fractions and analyze them with SDS-PAGE.
Note: Store the eluted fractions at 4°C. If -20°C storage is required, add glycerol to the fractions. For long-term storage, add protease inhibitors to the fractions.
If one wishes to reuse the resin or pre-packed column to purify the same or different recombinant protein, wash the resin/column with 1 M NaOH for 30 minutes and equilibrate the resin/column in a suitable binding buffer.