



Kinetic characterization of protein-ligand binding by surface plasmon resonance (SPR)
Surface Plasmon Resonance (SPR) is a phenomenon that can be observed in thin conducting films at an interface between media of different refractive index. In SPR systems, the media are the glass of the sensor chip and the sample solution, and the conducting film is a thin layer of gold on the sensor chip surface. SPR is a physical process that can occur when plane-polarized light hits a thin metal film under total internal reflection conditions. When a light beam hits a half-circular prism, the light is bent towards the plane of interface, when it is passing from a denser medium to a less dense one. Changing the incidence angle (Θ) changes the out-coming light until it reaches a critical angle. At this point, all the incoming light reflects within the circular prism. This is called total internal reflection (TIR). The surface is coated with a thin film of a noble metal, usually gold. Gold is used in sensor chips because it combines favourable SPR characteristics with stability and a high level of inertness in biomolecular interaction contexts. At a certain combination of angle of incidence and energy (wavelength), the incident light excites plasmons (electron charge density waves) in the gold film. As a result, a characteristic absorption of energy via the evanescent wave field occurs and SPR is seen as a drop in the intensity of the reflected light as shown in figure 1.
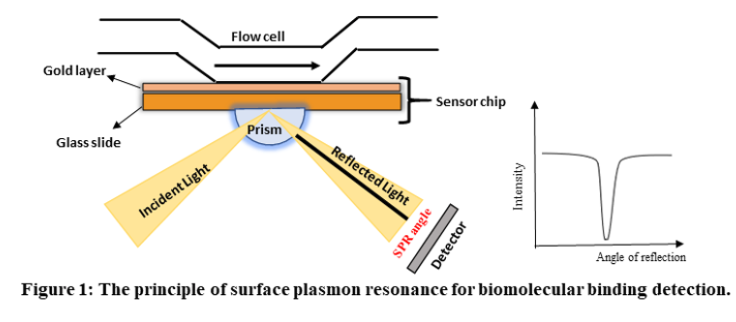
In an SPR experiment, one molecule (the Ligand) is immobilized on a sensor chip and binding to a second molecule (the Analyte) is measured under flow. Response is measured in resonance units (RU) and is proportional to the mass on the surface, and for any given interactant, the response is proportional to the number of molecules bound to the surface. Response is recorded and displayed as a sensogram in real time.
SPR instruments comprise three essential units integrated into one system: sensor surface, liquid handling (microfluidic) unit, and the optical unit.
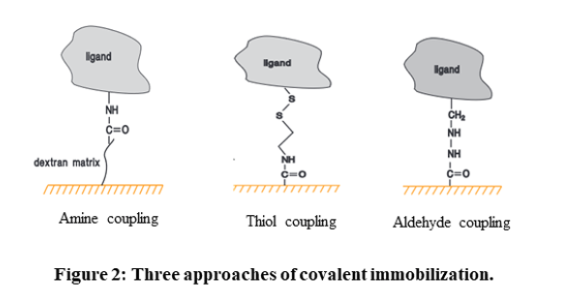
High-affinity capture: The molecule of interest is attached to another molecule through non-covalent interactions. This other molecule is usually attached to the sensor chip surface through covalent immobilization.
Hydrophobic adsorption: It exploits hydrophobic interactions to attach either the molecule of interest or a hydrophobic carrier such as a lipid monolayer or bilayer to the sensor chip surface.
Microfluidic system: The microfluidic system of SPR consists of an integrated flow cell or IFC, through which the sample containing the analyte is supplied to the sensor surface in a controlled fashion. The delivery of sample and buffer to the flow cells is done with extreme precision through the pump system and valves in the IFC. This ensures that a continuous flow of liquid is maintained over the sensor surface during analysis, switching between buffer and sample with minimal disturbance or dispersion of the sample boundary. The precision in sample delivery plays a crucial role in ensuring the reproducibility of assay procedures and provides the controlled conditions necessary for interpreting kinetic data obtained from the interaction studies.
Optical system: As mentioned earlier, the optical system involves a prism-coupled configuration that is commonly used in SPR experiments. This optical system comprises various components, including: