
Qualitative determination of retained ausenite content in the heat treated steels is possible by X-ray diffraction technique. One of the basic approach is by comparing integrated intensities.
The expression for the intensity diffracted by a single phase powder specimen in a diffractometer is given by:
`I = ((I_0e^4)/(m^2c^4))((lambda^3A)/(32pir))(1/nu^2)[|F|^2p((1+cos^2theta)/(sin^2theta+))](e^(-2M)/(2mu))`
Above equation can be written as:
`I = (K_2R)/(2muM)`
where,
`K_2 = ((I_0lambda^3A)/(32piR))[(mu_0/(4pi))^2(e^4/m^2)]`
`R = (1/nu^2)[|F|^2p((1+cos^2theta)/(sin^2theta+))e^(-2M)]`
`K_2` depends only on incident beam, but not on crysal structure.
`R` depends only on crystal structure, but not on surrounding phase.
`2mu` depends on both vrystal structure and surrounding phase.
Thus for Austenite (`gamma`) and Martensite (`alpha`) above euation becomes:
`I_gamma = (K_2R_gammaC_gamma)/(2muM)`
`I_alpha = (K_2R_alphaC_alpha)/(2muM)`
From these two equations:
`I_gamma/I_alpha = (R_gammaC_gamma)/(R_alphaC_alpha)`
In this equation we have to caluclate `C_gamma/C_alpha` to evaluate reatined austenite.
`I_gamma` and `I_alpha` are the integrated intensities for each of the diffraction line (area unde the curve after background substraction).
By considering `C_gamma+C_alpha=1`, we can find out the actual amount of each phase. By considering several pairs of austenite and matensite lines, we can obtain several values of retained austenite content.

You would be provided with two X-ray diffraction patterns of steels. On which, one is water quenched (Figure 1) and anthoer one is liquid nitrogen quenched (Figure 1). Based on the theory, calcualte retained austenite (`C_gamma`) for below mentioned pairs of austenite and martensite lines.
`gamma_200-alpha_200`
`gamma_220-alpha_200`
`gamma_200-alpha_211`
`gamma_220-alpha_211`
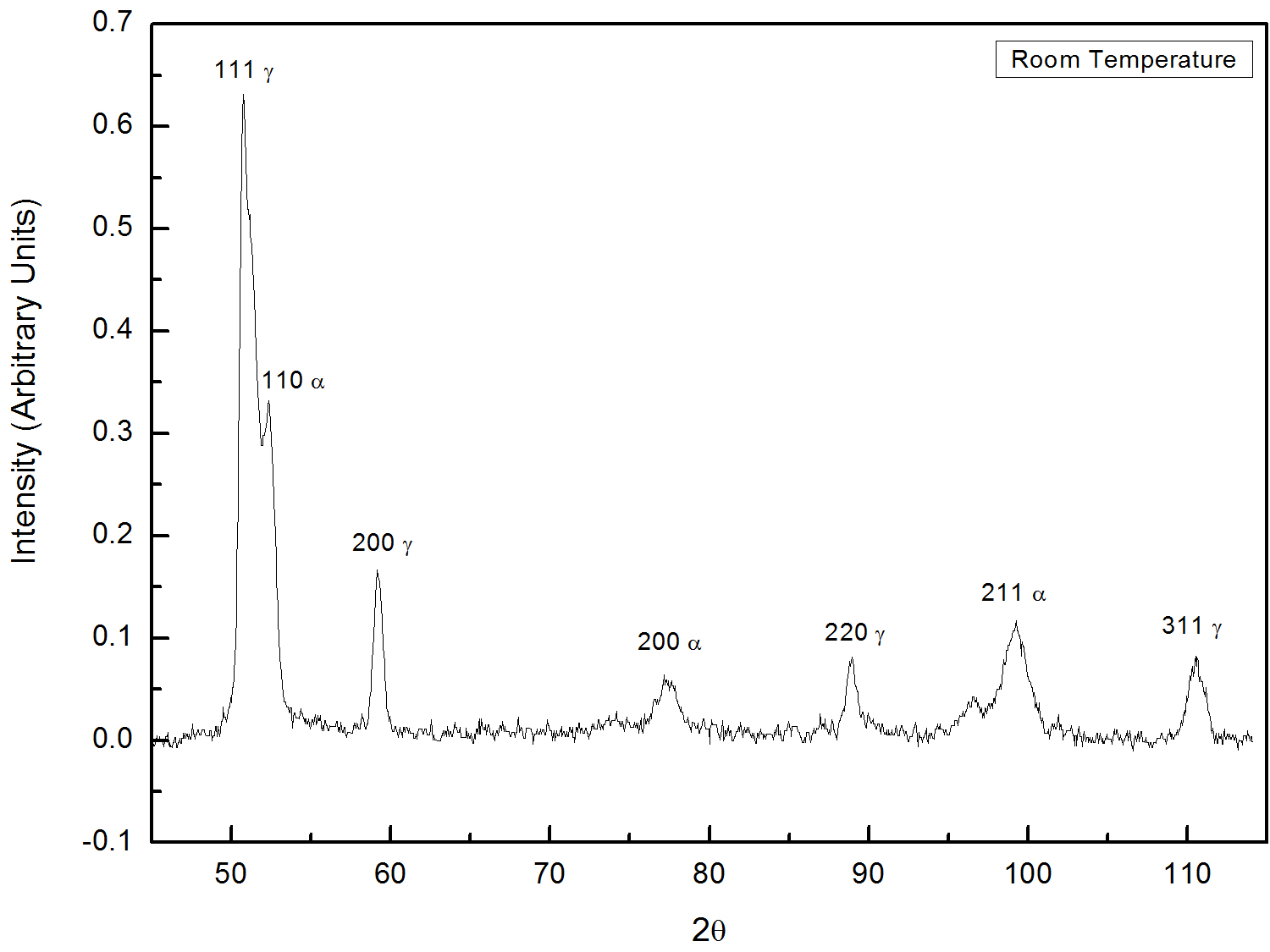
Fig. 1: XRD pattern taken from water quenched steel.
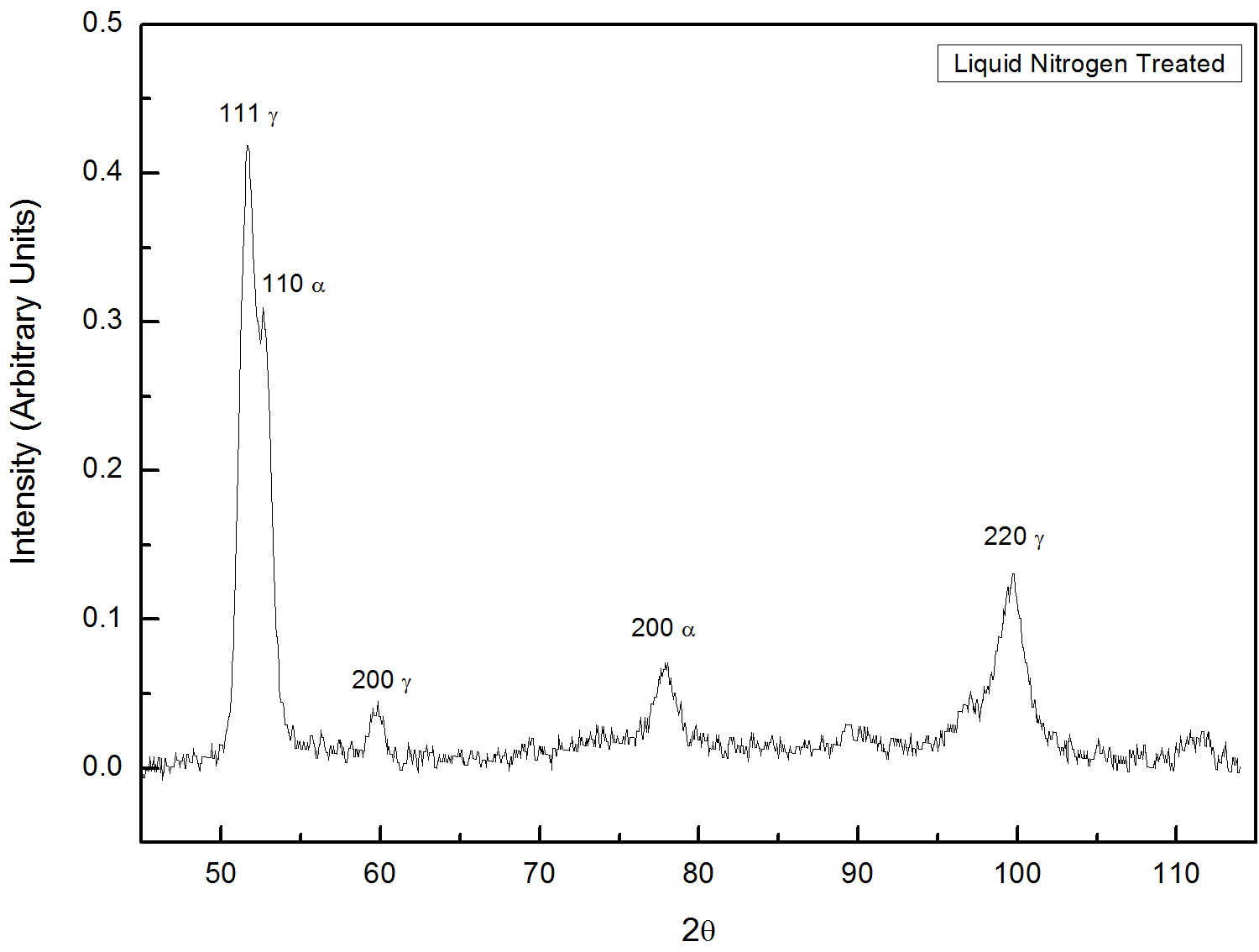
Fig. 2: XRD pattern taken from liquid nitrogen quenched steel.

Based on the theory, the retained austenite (`C_gamma`) for both steel samples have been calculated, and the corresponding results are tabulated below.
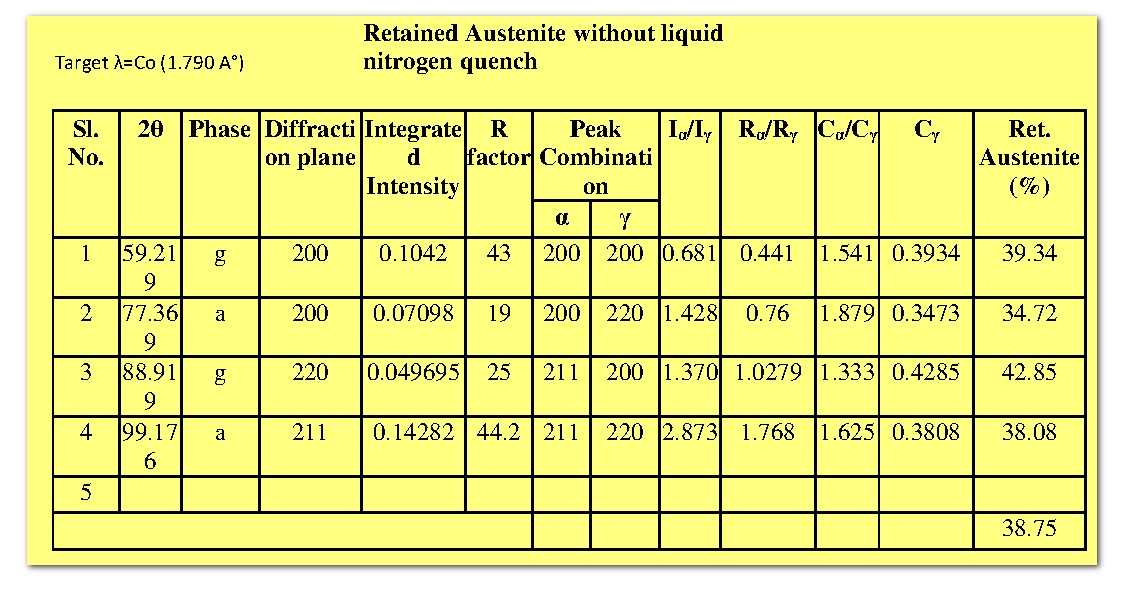
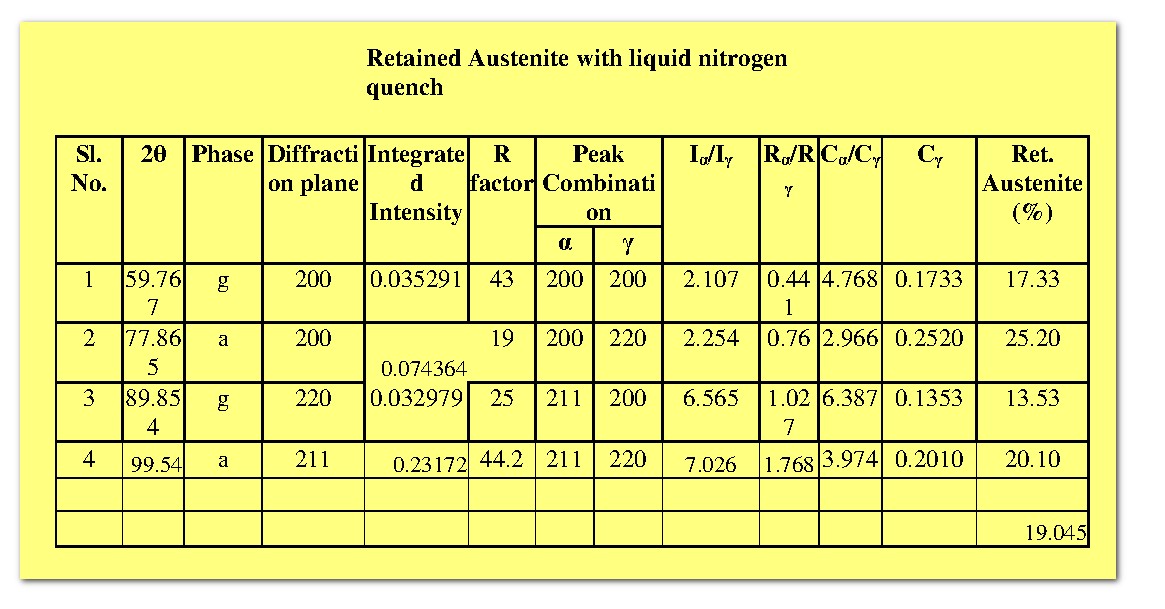
It is found that the fraction of martensite is greater when quenched in liquid nitrogen than at room temperature. This observation is well expected, because the martensite content in the steel is depends on cooling rate.

- B. D. Cullity, Elements of X-Ray Diffraction, 2nd Ed. Addison-Wesley, 1978.
